The RNA Revolution
Banner Image: A computational model of Double-stranded RNA. Nucleic acid builder module of AMBERTools has been used to make this structure. (Source: Wikipedia)
—written by Shanzeh Mumtaz Ahmed, MSc
The COVID-19 pandemic made ribonucleic acid, or RNA, a household name. While–as any biology student can attest to–RNA is neither a recent term nor an obscure concept, the successful production of COVID-19 vaccines and subsequent global attention heralded in the RNA revolution. Decades of research into this fundamental molecule has allowed scientists to co-opt the natural ability of cells to fight off pests and diseases.
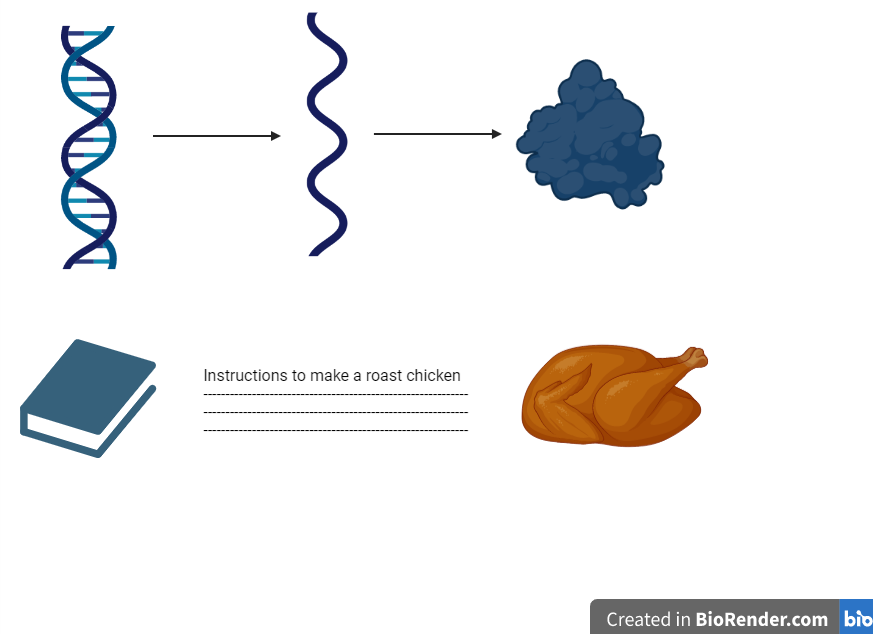
Like DNA (deoxyribonucleic acid), RNA is a foundational biological molecule that carries genetic information. This information, held in genes on the DNA, is copied into RNA, which is then used by a cell’s machinery to build proteins needed for the body to carry out its functions. In this way, RNA acts as a messenger of sorts, doing much of the heavy lifting in keeping our biological systems going.
Dr. Julie Claycomb, an Associate Professor in the Department of Molecular Genetics at the University of Toronto, describes DNA as the “family cookbook which has all the information to make numerous wonderful feasts,” while the messenger RNA (mRNA) is more of a portable “individual recipe card” used to make the final dish. Easy access to the right recipe, or the right parts of the DNA, allows your body to build the proteins that you need.
There are different types of RNA that perform different functions. For example, mRNAs are involved in protein synthesis, whereas small RNAs do the opposite by turning off protein production. This ability to control what proteins a cell can produce is what gives RNA enormous potential in revolutionizing advancements in health, agriculture and beyond. RNAs can be poked and prodded, tweaked and engineered, to improve treatments for different diseases or manage pests and crop yields on a farm.
RNA in Healthcare
“We understand the design,” says Dr. Amy Buck, a Professor of RNA and Infection Biology from the University of Edinburgh. Dr. Buck studies how small RNAs impact and regulate immune responses. She explains that it is our familiarity with RNA that makes it such a valuable drug.
She thinks of RNA as a Lego set. In understanding how the pieces fit together, we have the capacity to arrange them in different ways to create a range of products for therapies or vaccines. With advances in technology, designing RNAs has become more sustainable, affordable and innovative.
In fact, the COVID-19 pandemic exhibited, in real-time, the versatility of RNA technology with multiple vaccines being approved worldwide. If mRNA is like a portable recipe card, then for vaccine synthesis, imagine you get to write the recipe. mRNA can be designed to generate a small portion of a viral protein, packaged (often in a fat-based circular particle, called a lipid nanoparticle), and introduced to your body. Once inside, your cells can take up this particle and “read” the mRNA sequence. The cell’s machinery is then able to build that small piece of viral protein. Immune cells interact with these proteins and can quickly identify the live virus upon subsequent infection. That quick identification and clearance generally prevents you from experiencing severe illness.
This image depicts a 3D generated rendering of a whole influenza (flu) virus with a light grey surface membrane, set against a black background. The virus’ surface proteins, hemagglutinin (HA) and neuraminidase (NA), are depicted in light and dark blue, respectively. HA is a trimer, which is comprised of three subunits, while NA is a tetramer, which is comprised of four subunits, with a head region resembling a 4-leaf clover. Illustrator: Dan Higgins
There are many reasons we may want to “switch on” protein production. For example, to treat medical conditions where an individual’s body creates faulty versions of a necessary protein. Dr. Buck mentions cystic fibrosis, a condition in which the lungs accumulate mucus due to a specific faulty protein, as an example. In these cases, inhaled mRNA therapies can allow for the correct protein to be introduced to the lungs to compensate for the naturally defective ones.
RNA in Agriculture
Small RNAs are also useful in agriculture. Dr. Cei Abreu-Goodger, a Reader in Bioinformatics at the Institute of Ecology and Evolution from the University of Edinburgh, uses computers to investigate how small RNAs function, both inside and outside of the organisms that produce them.
He explains that gene silencing, or turning off protein production, was actually serendipitously discovered in plants in the 1990s. Scientists were attempting to use RNA technology to create more of a purple pigment in petunias and ended up discovering that they were doing the opposite and “silencing” the pigment, creating white flowers instead.
Unlike RNA technology in healthcare, you don’t need to deliver and target RNA into specific plant cells. Delivery can be as simple as spraying the designed RNA onto the outside of the plant, especially when the goal is to target external pests.
Of course, RNA is designable and can be targeted for more than pest prevention, including improving crop yields or preventing early ripening of fruits and vegetables, prolonging their shelf life. The more we know about how to customize an RNA sequence, the more versatile RNA technology can be.
To support this, plant biologists work with computational biologists like Dr. Abreu-Goodger, and collect information like the genetic sequences of various agriculturally relevant host species. This expanding database allows researchers to design their RNAs with more targeted effects on the specific plant species that they want to work with.
RNA Technology - Is This The Future?
RNA technology has been shown to be relatively affordable, biodegradable and re-designable, so why aren’t we using it all the time? Outside of the sequence itself, there are two key factors that influence the success of RNA technology: stability and delivery.
As any RNA researcher will tell you, an RNA molecule is not as stable as a DNA molecule. Like walking a tightrope, a delicate balance must be maintained.
Whether in the lab, in the body, or on a plant, the RNA sequence needs to be stabilised so that it does not fall apart or degrade before it gets to its target location. However, it cannot be so stable that it is not able to biodegrade in a timely manner after it has performed its function. For example, in the case of mRNA vaccines, this stability is on the order of days to a few weeks at most.
Even after a stable molecule is constructed, delivery can present another challenge. The RNA needs to target specific molecules on specific cells for it to successfully get taken up by the cells and perform its function. This is an area of active research and scientists are still trying to figure out ways to successfully deliver RNA to only the specific tissues where it is needed.
Despite these challenges, the future is promising.
Scientists are exploring the idea of using RNA technology to reprogram our immune cells to identify and target cancer cells. It’s like giving the immune system a map with the Xs already marked. Underlying all of these innovative new therapies is continued work towards understanding the natural ways RNA moves between cells. Researchers continue to accumulate foundational knowledge on the biological processes in which RNA is involved across different host species, from worms to humans. Like Drs. Claycomb, Buck and Abreu-Goodger, there are many more scientists across nations and disciplines who are continuing to explore the frontiers of RNA research, pushing this exciting revolution forward.